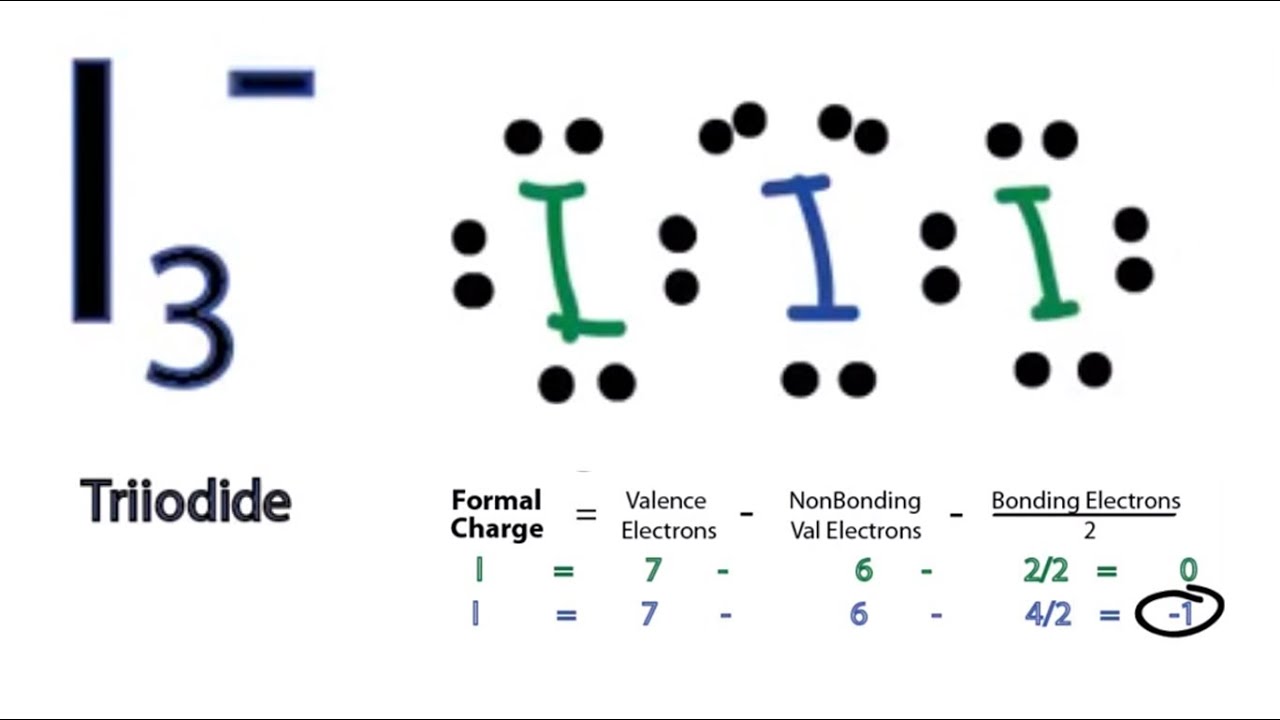
I3 Lewis Structure How to Draw the Lewis Structure for I3 YouTube
The triiodide ion (I3-) is an anion that is formed by combining three iodine atoms. It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. The ion has a linear shape, with the three iodine atoms arranged in a straight line. Drawing the Lewis Structure of I3-

to Chem Number of lone pair present at
For the I3- Lewis structure we first count the valence electrons for the I3- molecule using the periodic table. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. For I3- we'll end up with 6 additional valence electrons after filling the.
Trigonal Pyramidal Bond Angle
Triiodide in Chemistry usually refers to the Triiodide ion, I3- This anion, one of the polyhalogen ions, is composed of 3 iodine atoms and is formed by combining the aqueous solution of iodine and iodide salts. A few salts of the anion have been isolated, including ammonium Triiodide ( [NH4]+[I3]− and thallium (I) Triiodide (Tl+ [I3]−)).

Electron Geometry VS Molecular Geometry Difference between Electron
I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 + I- —-> I3- This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings.
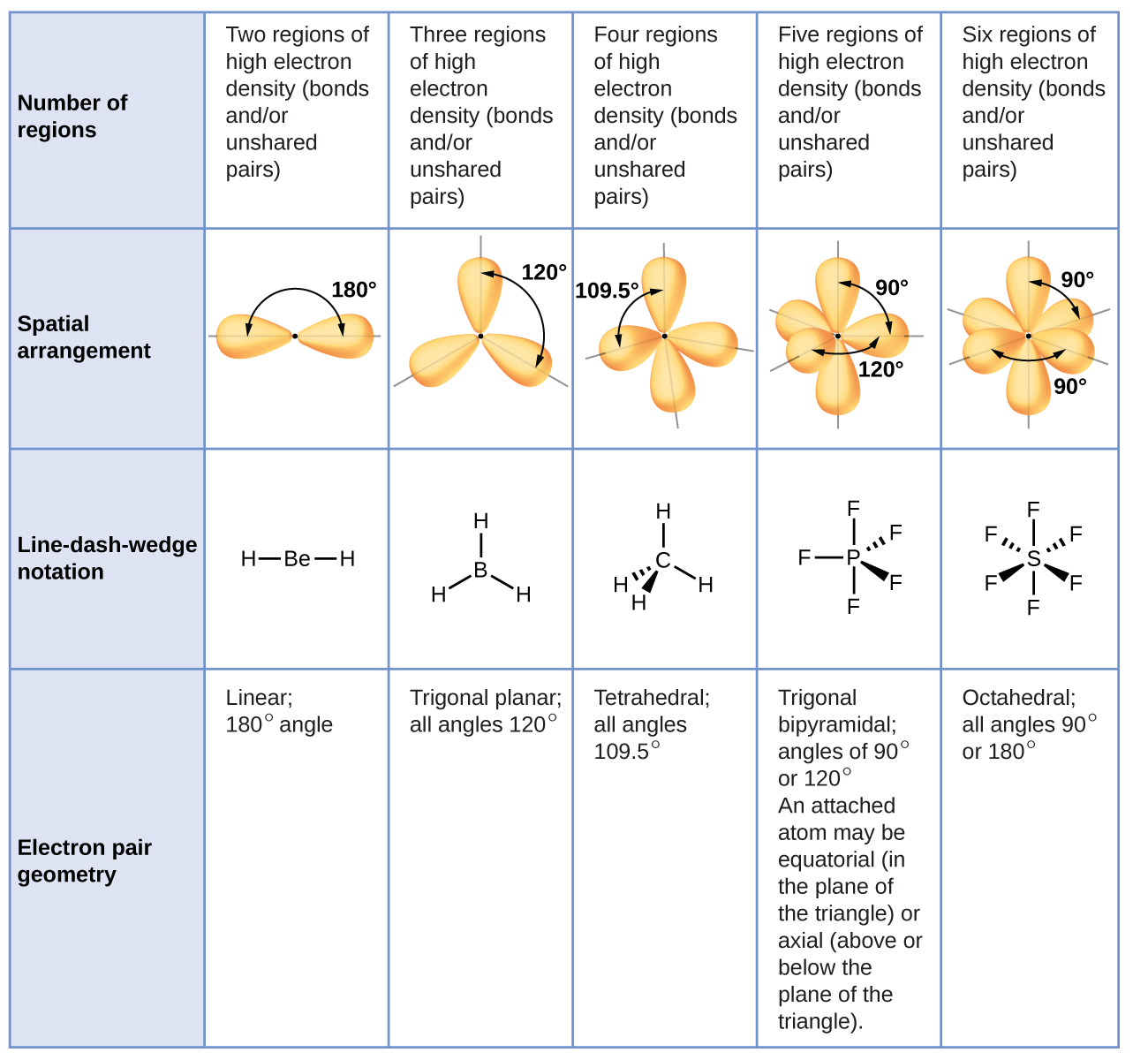
9.4 VSPER Theory Predicting Molecular Geometries Chemistry LibreTexts
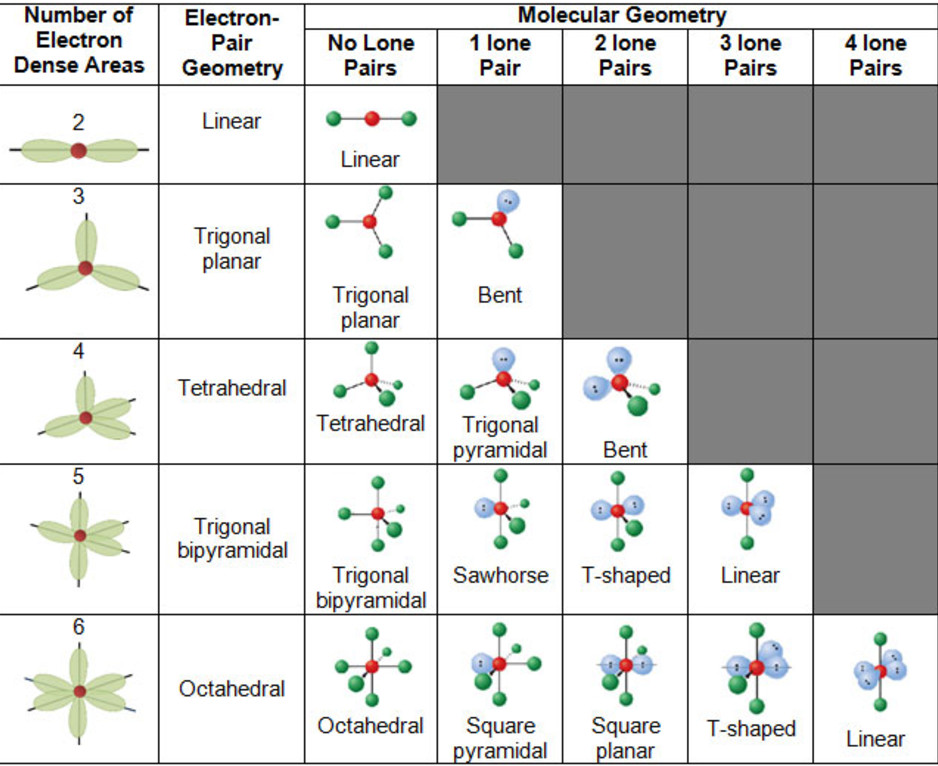
The molecular geometry of I3- takes into account the number of atoms attached to the central atom and the number of lone pairs on the central atom. The molecular geometry is key to understanding the physical and chemical properties of a molecule. In the case of I3-, the molecular geometry is linear.

I3 Molecular Geometry, Bond Angles & Electron Geometry YouTube
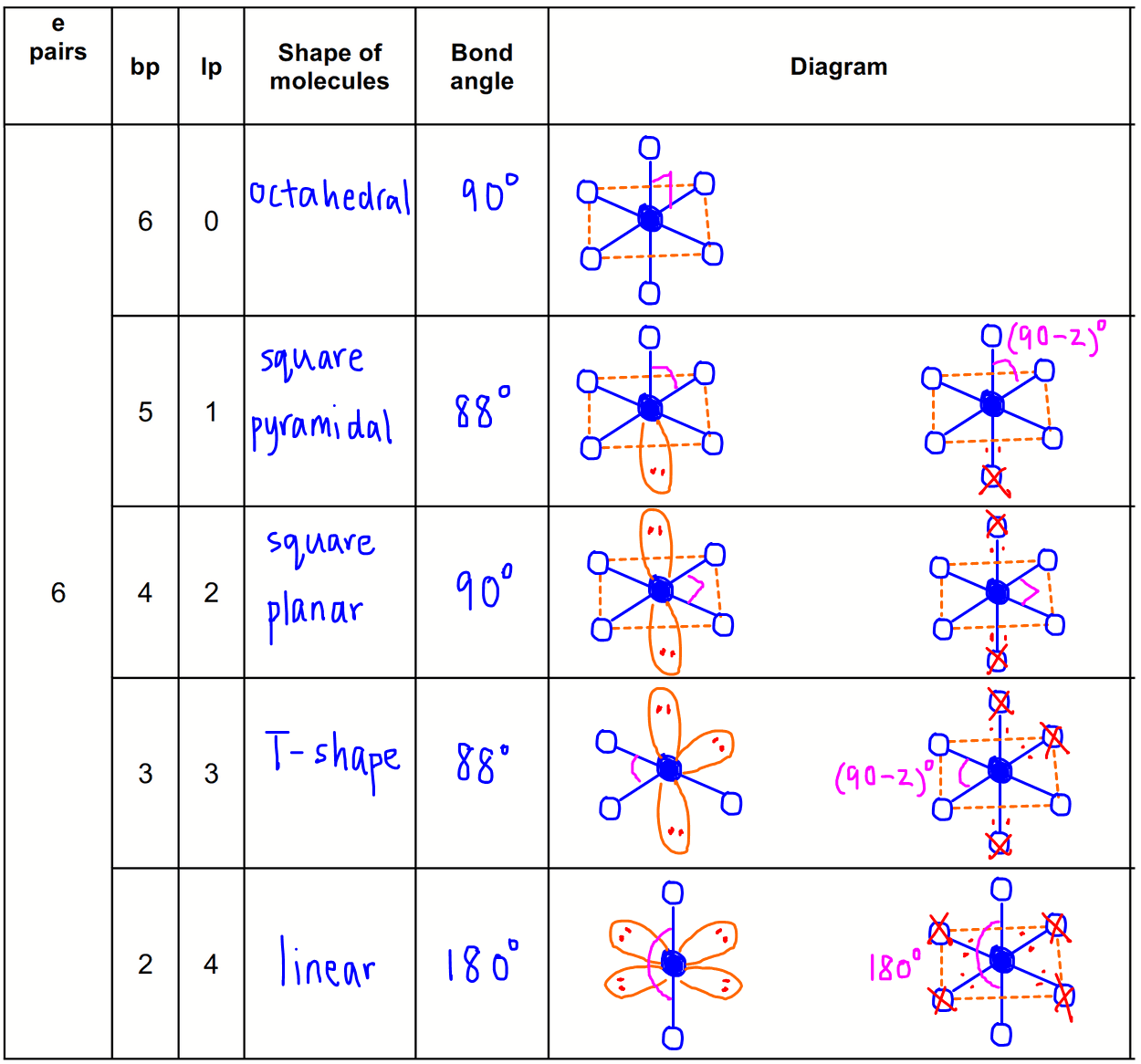
Figure 5.2. 11: (a) XeF4 adopts an octahedral arrangement with two lone pairs (red lines) and four bonds in the electron-pair geometry. (b) The molecular structure is square planar with the lone pairs directly across from one another. In a certain molecule, the central atom has three lone pairs and two bonds.

Electron Geometry Vs Molecular Geometry Chart
The i3 Lewis structure refers to the arrangement of atoms and electrons in a molecule of iodine triiodide (I3-). In this structure, three iodine atoms are bonded together, forming a linear molecule. The Lewis structure provides a visual representation of the valence electrons and the bonds between atoms.

I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
For the polyatomic ion I3-:a) Draw the Lewis structure from its constituent atoms.b) Predict the bond angle around one of the central atom.c) Draw the ion in.

I3 Molecular Geometry, Bond Angles & Electron Geometry (Triiodide Ion
Written by Priyanka in Lewis Structure It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the molecule. Today we are going to go through the Lewis structure of I3- or also know as Triodide ion as it has a negative charge on it.
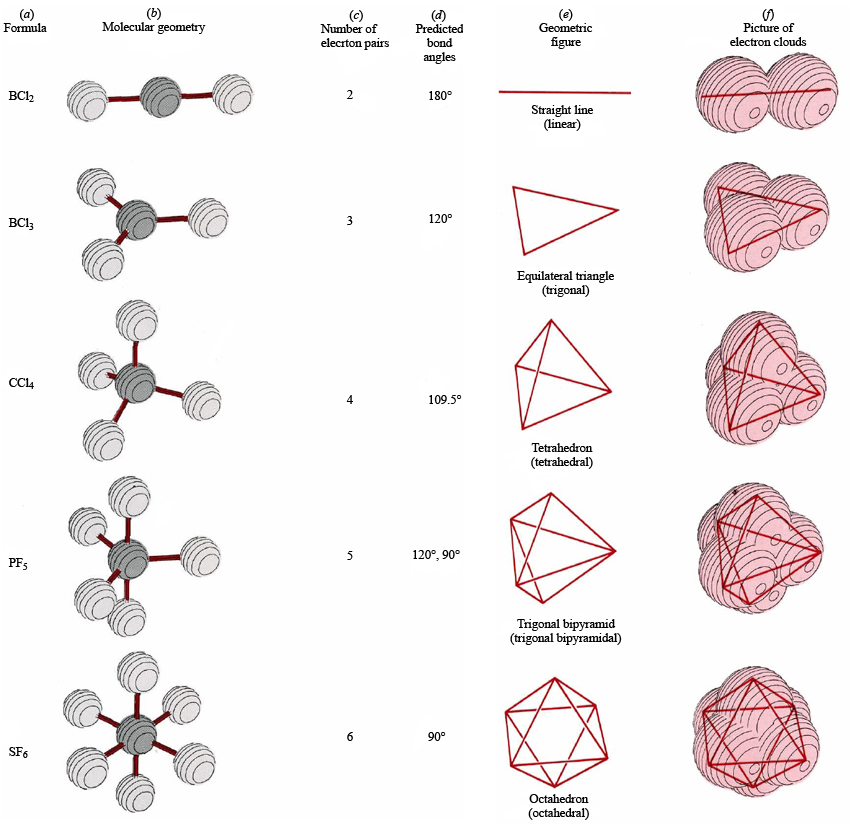
Molecular Geometry Chemistry Socratic
Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. With two bonding pairs on the central atom and no lone pairs, the molecular geometry of CO 2 is linear (Figure 6.3.3 ). The structure of CO 2 is shown in Figure 6.3.1. 5. If someone asked what the hybridization on the C atom was, we would first draw the Lewis.
Electron
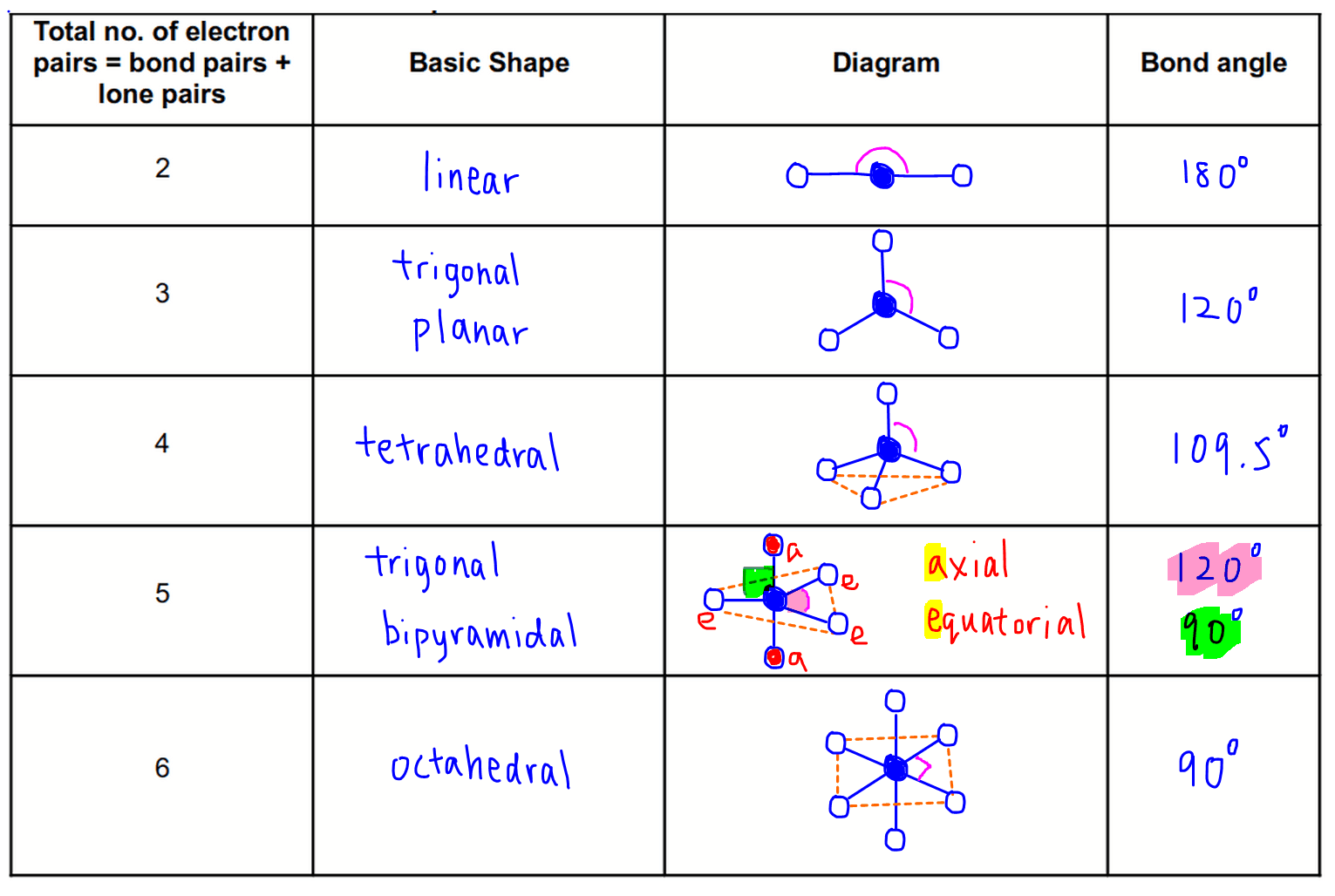
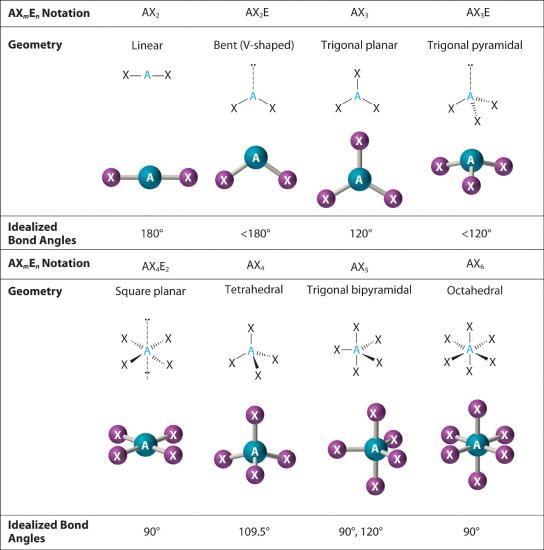
Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.

Pin on Lưu nhanh
You will also learn interesting facts about the molecular geometry or shape of [I3]-, its electron geometry, bond angle, hybridization, formal charges, and polarity nature. So, without any further delay, dive into the article and gain some valuable chemistry knowledge. Page Contents show How to draw lewis structure of I3-?
3.6 Predicting the Geometry of Molecules Chemistry LibreTexts
An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.

Gallery For > I3 Lewis Structure Molecular geometry, Vsepr theory
This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the triiodide ion..

Chapter 5.1 Predicting the Geometry of Molecules Chemistry LibreTexts
Three iodine atoms in a row, with the central atom having two bonding pairs and three lone pairs of electrons. The ion is a classic AX2E3 shape according to.
Socl2 bond angle 🌈BF3 (Boron trifluoride) Molecular Geometry, Bond
Hello Guys! Welcome back to our channel and today in this video we will help you determine the molecular geometry of I3- ion(Triiodide ion). It consists of t.