
What is the a) molar enthalpy of the ammo... Physical Chemistry
Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in NH4ClO4: Molar Mass (g/mol) N (Nitrogen) 1 × 14.0067 = 14.0067. H (Hydrogen) 4 × 1.00794 = 4.03176. Cl (Chlorine) 1 × 35.453 = 35.453.

Molar Mass of NH4Cl (Ammonium chloride) YouTube
NH4Cl molecular weight. Molar mass of NH4Cl = 53.49146 g/mol. This compound is also known as Ammonium Chloride. Convert grams NH4Cl to moles. or. moles NH4Cl to grams. Molecular weight calculation: 14.0067 + 1.00794*4 + 35.453. Percent composition by element. Element: Chlorine Symbol: Cl Atomic Mass: 35.453

How to find the molecular mass of NH4Cl (Ammonium Chloride) YouTube
Ammonium Chloride. IUPAC identifier. Office of Data and Informatics. IUPAC Standard InChI: IUPAC Standard InChIKey:NLXLAEXVIDQMFP-UHFFFAOYSA-N. CAS Registry Number: This structure is also available as a. Species with the same structure: (NH4)Cl; Ammonium chloride ( (NH4)Cl); Amchlor; Ammoneric; Ammoniumchlorid; Ammonium muriate; Chlorid amonny.

How Do You Calculate The Molar Mass Of Glucose?
Ammonium Chloride NH4Cl Molecular Weight, molar mass converter. ENDMEMO. Home » Chem » Compound Search; Ammonium Chloride. Name: Ammonium Chloride. Formula: NH4Cl. Molar Mass: 53.4915. NH4Cl Molar Mass Converter. Weight: Mole: Example Reactions: • NH3 + HCl = NH4Cl • NH4Cl + NaOH = NH3 + H2O + NaCl • NH4Cl + KOH = NH3 + H2O + KCl.
Al molar mass htpastor
35.45. Total Mass. 53.489. The molar mass of NH4Cl (ammonium chloride) is: 53.489 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).
Molar Mass / Molecular Weight of NH4Cl Ammonium chloride YouTube
Explanation of how to find the molar mass of NH4Cl: Ammonium chloride.A few things to consider when finding the molar mass for NH4Cl:- make sure you have the.

Ammonium chloride NH4CL mole to weight YouTube
Do a quick conversion: 1 grams NH4Cl = 0.01869457292809 mole using the molecular weight calculator and the molar mass of NH4Cl. Convert grams NH4Cl to moles - Conversion of Measurement Units.. Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams.
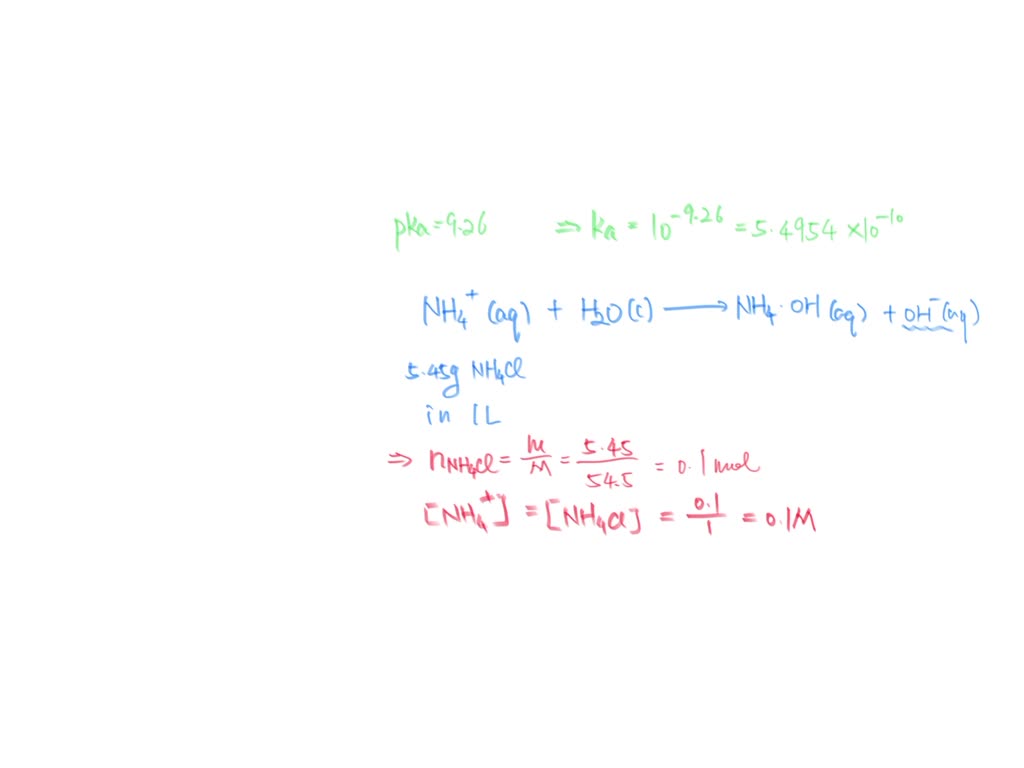
SOLVED Given the pKa for ammonium ion is 9.26, what is the pH of 1.00
Hence the Molar mass of NH4Cl is 53.489 g/mol. I hope you have understood the short and simple calculation for finding the molar mass of NH4Cl. Remember. In some books, you may see the unit of molar mass as grams/mole or g/mole. But all these units (i.e g/mol, grams/mole and g/mole) are the same.

molar mass of (NH4)3P YouTube
NH4Cl Molar Mass NH4Cl Oxidation Number. Thermodynamics. Thermodynamics of the reaction can be calculated using a lookup table. Choose Compound States. No state of matter options are available for this reaction. Enthalpy Calculator. Is the Reaction Exothermic or Endothermic? NH 4 (s carbamate) 2 mol-645.04728 kJ/mol: 1290.09456 kJ: Cl 2 (g)

10g of NH4Cl (molar mass 53.5 gm mol^1 ) when dissolved in 1000 gm
Ammonium chloride is a white crystalline solid. It is soluble in water (37%). The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. It is used to make other ammonium compounds, as a soldering flux, as a fertilizer, and for many other uses.

c2h5oh molar mass
Calculate the molar mass of the following:NH4Cl (Ammonium Chloride)SUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34WdYMOm47PkWov.

Nh4cl Molar Mass
CAS #: 12125-02-9 EC Number: 235-186-4 Molar Mass: 53.49 g/mol Chemical Formula: NH₄Cl Hill Formula: H₄ClN Grade: ACS,ISO,Reag. Ph Eur. View Products on Sigmaaldrich.com. 101145 View Pricing & Availability. Download Product Safety Card; Recommended Products. Overview; Supporting Documentation.

Ammonium chloride NH₄Cl Molecular Geometry Hybridization
As mass / volume = molarity × molar mass, then mass / (volume × molar mass) = molarity. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 × 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Simply type in the remaining values and watch it do all.
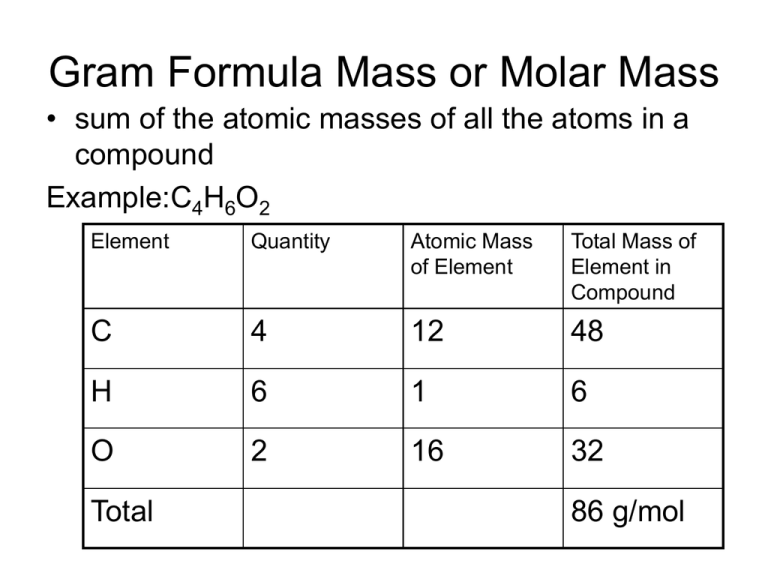
Gram Formula Mass or Molar Mass
Ammonium chloride is an inorganic chemical compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac.The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of.

Solved Mole Conversions What is the molar mass of 1. NH4CL
Calculate the molecular mass of the following:NH4Cl (Ammonium Chloride)SUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34WdYMOm47P.
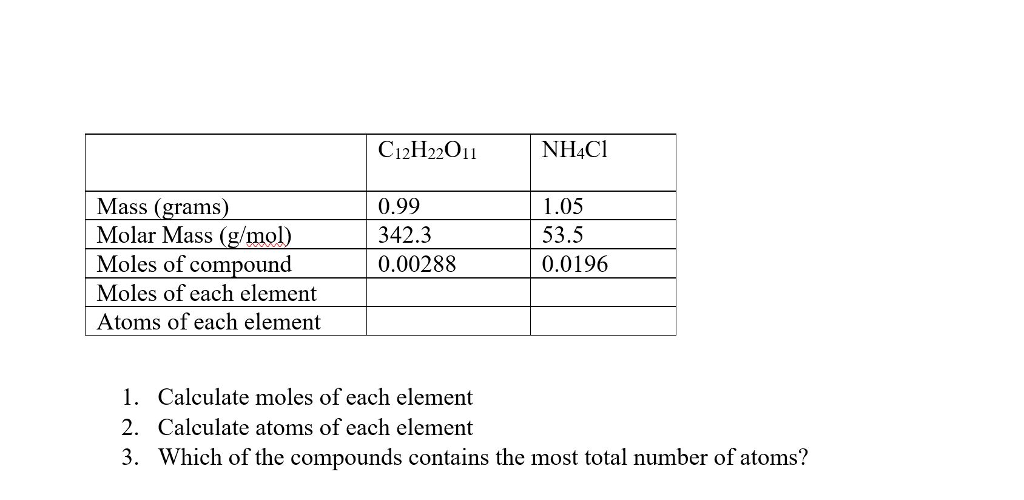
Solved Calculate Moles Of Each Element Calculate Atoms Of...
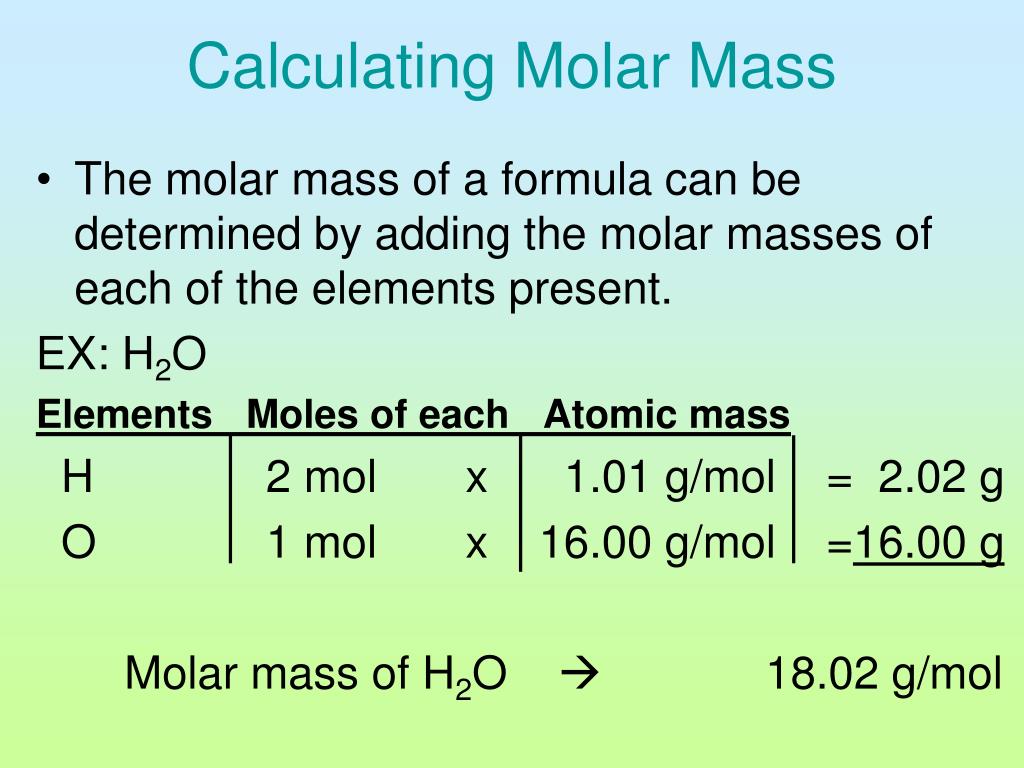
Example: calculating molar mass. Let's calculate the molar mass of carbon dioxide (CO 2): Carbon (C) has an atomic mass of about 12.01 amu. Oxygen (O) has an atomic mass of about 16.00 amu. CO 2 has one carbon atom and two oxygen atoms. The molar mass of carbon dioxide is 12.01 + (2 × 16.00) = 44.01 g/mol. Lesson on computing molar mass